Categories
YHLO - YHLO GLINE-2019-nCoV Ag Rapid Antigen Self-test
Price:
HK$ 100
Product
Code:A17090
The YHLO government approves the rapid test kit for novel coronavirus antigens. This rapid COVID-19 virus rapid test produces highly accurate results in as little as 15 minutes.
Description
✦Product details
| - |
Recognized by the Hong Kong government and produced by the testing center BGI designated production plant |
| - |
Obtained EU CE certification and included in the list of EU-approved 2019 coronavirus rapid test kits |
| - | ISO9001 and ISO13485 certification |
| - | Clinically validated sensitivity of 96.38% and specificity of 99.56% |
| - |
Detection of SARS-CoV-2 antigen using nasopharyngeal swab samples to assist in assessing the status of novel coronavirus infection |
| - | The operation is very simple, only 15 minutes to get test results |
| - | Suitable for self-testing at home, school, workplace, medical center, etc. |
✦Materials Provided:
1. Test Cassette x1
2. Swab x1
3. Sample Extraction Tube x1
4. Biohazard Sample Bags x1
5. Instructions for Use x1
Operation step (Press
 to watch video)
to watch video)✦TEST PROCEDURE
1. Peel off aluminum foil seal from the top of the extraction tube containing the extraction buffer. Place it on the package.
2. Open swab package and take the swab out. NOTE: Keep fingers away from swab end.
3. To collect a nasal (anterior) swab sample, carefully insert the swab into the nostril until you fell a slight resistance (about 1.5cm up your nose). Using gentle rotation, roll the swab firmly around the inside of the nostril, making 5 complete circles each nostril, and then slowly remove it while wiping.
4. Place the swab into the sample tube and then completely immerse the swab head in the sample. Vigorously mix the solution by rotating the swab forcefully against the side of the tube at least 10 times (while submerged).
Squeeze the tube 5 times by hand to ensure that the sample on the sampling swab is fully eluted into the sample extraction buffer. Snap the swab head and keep it in the tube.
Press the nozzle cap tightly onto the tube.
5.Open pouch and place the card on a clean, dry, flat surface.
6. Apply 3 drops of extracted sample to the specimen well of the test card.
7. Wait 15 minutes. NOTE: Do not disturb card during this time. False results can occur if the card is
disturbed/moved or test results are read before 15 minutes.
8. Interpret the test results at 15~20 minutes. Do not interpret the results after 20 minutes. The kit components and patient samples should be handled as infectious waste. The kit components must be disposed of in accordance with local disposal regulations.
......................................................................
Result Interpreation
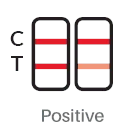 |
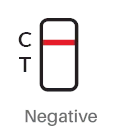 |
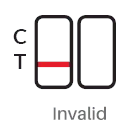 |
||
| POSITIVE: The presence of two lines at control line (C) and test line (T) indicates a positive COVID-19 test result (even if one of the lines is faint) | NEGATIVE: The presence of only the control line (C) indicates a negative COVID-19 test result. |
INVALID: If there is no control line (C) after performing the test, the result is invalid |
||
INTERPRETATION OF TEST RESULTS
1. Positive: Both red/purplish test band (T) and red/purplish control band (C) appear in window.
2. Negative: Only the red/purplish control band (C) appears in window. The absence of a test band (T)
indicates a negative result.
3. Invalid: There should always be a red/purplish control band (C) in the control region regardless of test
result. If control band (C) is not seen, it indicates that the incorrect operation process or the kit
has deteriorated or damaged. In this case, read the instructions carefully again and test with a new
test card. If the problem persists, contact your local supplier.
✦Origin: China
✦Storage: the product should be stored at 2-30°C, dry and out of light, it is validity for 18 months. Each test cassette should be used within 1 hour of as opening the sealed foil pouch. The test should be used in a room temperature (20-30°C) environment with a humidity of no more than 75%. If the test has been stored in a refrigerator, allow the test to equilibrate to room temperature for 30 minutes before use. The test should be used immediately after being removed from its sealed foil pouch.
✦Manufacture date (MFD) and Expiry date (EXP): marked on the label.
✦PRECAUTIONS
1.For in vitro diagnostic use only.
2.This test has been authorized only for the detection of proteins from SARS-CoV-2, not for any other virus or pathogen.
3.Use immediately after opening the pouch containing the test cassette.
4.Avoid touching any bleeding areas of the nostril area during specimens collection, as excess blood or mucus on the swab may interfere with the test and yield a false result.
5.Do not use if the test device package is damaged.
6.Do not use the kit contents beyond the expiration date.
7.Do not eat, drink, or smoke in the area where the specimens and kit contents are handled.
8.Do not interchange kit contents from different lots.
9.Do not make any decision of medical relevance without first consulting your medical practitioner.
10.Clean and disinfect all spills of specimens or buffer using a suitable disinfectant.
11.If the extraction buffer makes contact with the skin or eye, wash/ flush with a large volume of water. If skin irritation, rash or other abnormal reaction occurs, please get medical advice/attention.
12.Follow the instruction strictly when performing a self-test. Children and elders please use the test under the guardian assistance

